Oral nicotine products deliver nicotine, flavours and other chemicals to the mouths of people who use them. This section describes the chemicals found in non-therapeutic oral nicotine products, including:
It also discusses:
Oral nicotine products deliver nicotine to a person via sucking, chewing, or holding the product in the mouth. They include nicotine pouches, gum, gummies (sugar-coated lollies), discs (chewable tablets) and toothpicks (see Section 18C.1.1). Note that non-therapeutic oral nicotine products are distinct from approved therapeutic oral nicotine replacement therapy products (see Section 7.16) and from smokeless tobacco products (see Section 12.2.9).
Studies of the chemicals in non-therapeutic oral nicotine products have primarily tested nicotine pouches. There are numerous independent (non-industry) studies of the chemicals in nicotine pouches, but the biomarkers of exposure to these chemicals have only been studied by the tobacco companies that sell these products.
18C.4.1 Nicotine, synthetic nicotine and nicotine analogues
18C.4.1.1 Nicotine levels in oral nicotine products
Nicotine is a highly addictive drug and has been the common component of oral nicotine products. A study of 37 different oral nicotine pouches sold in the US in 2019 found that the total nicotine content ranged from 1.29 to 6.11 mg/pouch.1 A German study of 44 different nicotine pouches found a median of 9.48 mg of nicotine per pouch.2 Two of these pouch types contained over 40 mg of nicotine per pouch. Labelling of the nicotine content in these products was often poor. For 29 of these brands, an arbitrary descriptor (such as ‘easy’ or ‘ultra’) was used to indicate the nicotine content, rather than mass of nicotine. Four of the 44 brands tested contained no indication of nicotine levels.2 A study of Verve oral nicotine discs (chewable tablets) found 1.68 mg nicotine per tablet, compared to the labelling that indicated 1.5 mg was present. 3 Over 80% of this nicotine was liberated from the tablet after use,3 similar to the proportion of nicotine released from pouches.4 Nicotine levels in nicotine lozenges, chewing gum and toothpicks were found to be lower than pouches in one study.5
18C.4.1.2 Free-base nicotine and nicotine salts
Differing forms of nicotine in tobacco products and e-cigarettes
Nicotine in tobacco products can be found in a ‘free-base’ form or a protonated form (also called ‘nicotine salts’). A higher pH (lower acidity) leads to a higher ratio of free-base nicotine to nicotine salts. Historically, it was predicted that during smoking, free-base nicotine was more effectively delivered to the lungs and taken up into the blood.6 However, it was subsequently shown that uptake of nicotine into the blood from cigarettes occurs at similar rates for free-base and protonated nicotine.7,8 See Section 12.4.3.1 for more information.
E-cigarettes also contain differing forms of nicotine. There is some evidence that addition of acids to e-liquids, such as benzoic acid, will produce nicotine salts that are more readily taken up into the blood stream than free-base nicotine.9 See Section 18.5.3.1 for more details.
The nicotine in smokeless tobacco enters the body via the buccal mucosa (lining of the mouth) as opposed to the lungs. Unlike tobacco smoke, there is evidence that smokeless tobacco with a higher pH (less acidic), that contains a higher ratio of free-base to protonated nicotine, has a faster delivery of nicotine to the blood across the buccal mucosa.10,11
Differing forms of nicotine in oral nicotine products
Studies have shown that oral nicotine products with a higher pH generally have a higher ratio of free-base to protonated nicotine. The majority of studies to date on oral nicotine products have examined the forms of nicotine in pouches. In a study of 37 different pouch types, brands such as On! (made by Altria) and White fox (made by GN Tobacco) had the highest pH levels, close to 10.1 These brands contained over 95% of their nicotine in the free-base form. Velo and LYFT brands (made by British American Tobacco), on the other hand, had a pH closer to 7 and less than 50% of nicotine in free-base form.1 A similar association between pH and free-base nicotine was also found in a German study of 44 pouch types.2 There is also some evidence of acids such as benzoic acid added to some nicotine pouches, which may be creating nicotine salts.12
A study of different types of oral nicotine products found that pouches had the highest proportion of freebase out of total nicotine.5 Pouches ranged from 30.8% to 96.7% free-base nicotine with an average of over 50% of total nicotine being free-base. By comparison, nicotine chewing gums were over 30% free-base nicotine but toothpicks and lozenges were less than 10%.5
Nicotine salts have a smoother taste than free-base nicotine, possibly allowing for a higher concentration of nicotine in the pouch. To date, the consequences of differing forms of nicotine in pouches for nicotine uptake into the bloodstream and the potential for addiction are unknown. One study from the tobacco industry reported that blood nicotine levels were increased more slowly for pouch use than for cigarettes.13 In contrast, an independent study showed that the rate of nicotine uptake into the blood was similar for pouches and cigarettes.4
18C.4.1.3 Synthetic nicotine
Some oral nicotine products are described by manufacturers as containing synthetic nicotine and patents for synthetic nicotine are owned by companies that make oral nicotine products.
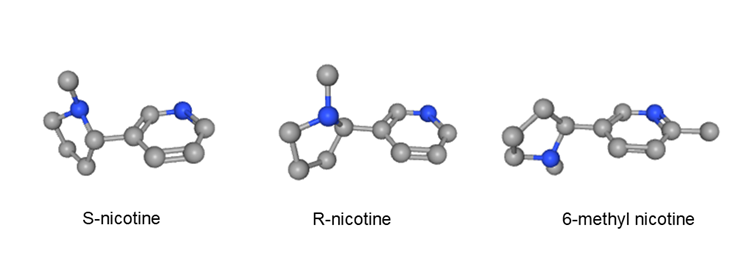
Nicotine is a molecule that has two different structural forms that are mirror images of each other, called S-nicotine and R-nicotine (see Figure 18C.4.1). These forms contain the same atom arrangement but differ in that one bond between atoms is rotated at a differing angle. Nicotine in tobacco plants and tobacco is over 99% S-nicotine. Pharmaceutical grade nicotine used in nicotine replacement products is also S-nicotine, purified to a high grade from tobacco plants.14 There is little known about the safety and health effects of R-nicotine in tobacco, although industry documents indicate that safety concerns were noted in the 1960s.14,15
Figure 18C.4.1 S-Nicotine, R-nicotine and 6-methyl nicotine molecules depicted in three dimensions by ball and stick models
Nicotine can also be chemically synthesised from non-tobacco starting ingredients, producing synthetic nicotine, which may also be called 'tobacco-free nicotine' (TFN). Early formulations of synthetic nicotine contained approximately half S-nicotine and half R-nicotine. Laboratory tests are able to differentiate synthetic and tobacco-derived nicotine, if the ratio of S- and R-forms is 50:50 in synthetic nicotine.15 However, there are synthetic nicotine products now made that contain over 99% S-nicotine, making it more difficult to differentiate between synthetic and tobacco-derived nicotine. Claims by manufacturers of using synthetic nicotine in their products are therefore difficult to assess. At least three companies own patents for the production of synthetic nicotine.16 Currently, synthetic nicotine, if purified into >99% S-nicotine, is said to be much more expensive to produce than natural nicotine.15,18
Some e-cigarettes and oral nicotine products are marketed as containing synthetic nicotine.14 This can be problematic for regulators in jurisdictions where tobacco products are defined as containing any part of the tobacco plant, not necessarily including synthetic nicotine (see Section 18C.8). While synthetic nicotine will not contain impurities that derive from tobacco plants, this does not mean that it is free of other impurities that could affect a person’s health. A study of impurities in nicotine solutions containing synthetic or tobacco-derived nicotine found similar levels of impurities in both, such as toxic lead and arsenic.19
One study has compared blood levels of nicotine in people who used oral synthetic nicotine (50:50 mix of S- and R-nicotine) versus those who used tobacco-derived 99% S-nicotine. The synthetic nicotine mix resulted in lower blood levels of nicotine from 15 to 90 minutes after use, but few obvious differences in user experience.20
There may also be confusion among consumers regarding terminology.21 Nicotine pouches and other products are often marketed as ‘tobacco-free’. But this does not mean that synthetic ‘tobacco-free nicotine’ was used to make them. Indeed, the presence of small amounts of tobacco-specific nitrosamines in many oral nicotine products2 (see Section 18C.4.5 below) indicates that the nicotine in these products was derived from tobacco. However, some oral nicotine products are marketed with claims of having synthetic nicotine, which are currently difficult to assess for accuracy.
18C.4.1.4 Nicotine analogues
An analogue is a molecule that is very similar to but not identical to another. Nicotine analogues have been found in e-cigarettes.22 Due to the molecular similarity, nicotine analogues have the potential to bind to nicotine receptors, eliciting similar responses to nicotine, such as addiction. There is evidence from internal tobacco industry documents that tobacco companies have been researching the effects of using nicotine analogues in their products for over two decades.23
Oral nicotine pouches and e-cigarettes have recently been sold that claim to contain a nicotine analogue called 6-methyl nicotine (see Figure 18C.4.1).22,24,25 The presence of this molecule has been confirmed in some e-cigarettes, including brands which do not include it in their labelling.22 Vendors selling oral nicotine and e-cigarettes containing 6-methyl nicotine do so using the trademarked names of Imotine and Metatine.25 6-methyl nicotine has also been referred to as HYT and hippotine.
Studies have shown that 6-methyl nicotine binds to nicotine receptors with binding affinity similar or stronger than nicotine.26,27 Independent and tobacco industry laboratory studies of cultured human bronchial cells showed that 6-methyl nicotine was more potent than nicotine at eliciting toxic effects to these cells.24,28,29 Rodent experiments have shown an increase in psychotropic activity in response to 6-methyl nicotine administration compared to nicotine.26 6-methyl nicotine is described as having acute toxicity to the skin and toxicity if swallowed, similar to nicotine.30
Some Zyn, Velo, On!, MG and Hippotine nicotine pouches contain 6-methyl nicotine according to their labels.25 These products ranged from 1.5 mg to 25 mg 6-methyl nicotine per pouch. However, the amounts of this molecule in these products have not been confirmed by scientific studies.25 The upper limit of this range is concerning, given 6-methyl nicotine’s potency of cellular toxicity compared to nicotine.
The use of nicotine analogues may allow the industry to circumvent regulations in some jurisdictions. Many countries have existing laws that regulate synthetic nicotine in tobacco products and e-cigarettes, but these may not include nicotine analogues.24,31 Since April 2022, the US Food and Drug Administration has been authorised to regulate nicotine produced by any source as a tobacco product, which includes synthetic nicotine. Products containing synthetic nicotine now require premarket authorisation from the FDA before sale. However, this ruling may not be applicable to nicotine analogues. Companies selling these products in the US claim that they are not subject to regulation by the FDA given that 6-methyl nicotine is not exactly the same as nicotine and is not made from nicotine.32 A letter in May 2024 from numerous public health authorities urged the FDA to regulate nicotine analogues either as tobacco products or as drugs.32
18C.4.2 Flavouring additives
Most oral nicotine products contain numerous flavouring chemicals. The names of these products often include flavour indicators such as bubblegum, cool mint, cinnamon, citrus, coffee, cola, vanilla and a range of fruit flavours. A US study classified the range of flavours into groups of tobacco, menthol/mint, fruit, drink, desert, aroma, spices and mixed.33 In this study, menthol/mint was the largest category. The range of attractive flavours and colourful packaging with cartoon images is likely designed to appeal to younger adults and adolescents.15
Flavouring chemicals that are heated or burned (in smoked tobacco or e-cigarettes) can undergo chemical reactions to produce toxic chemicals, but this risk is likely to be lower for oral nicotine products, as they are not heated.
One study of flavourings in pouches bought in Germany reported 13 substances that did not have authorisation to be used as flavourings in the EU. These substances included derivatives of menthol (see below in Section 18C.4.3), chemicals that were likely impurities in nicotine solutions or mint additives, as well as flavourings that are not authorised due to health concerns. This last category included limonene, citral, geraniol and isoeugenol (mostly as potential allergens).12 Another study of five brands of nicotine pouches found a mean of 18 flavouring chemicals per pouch.34 The range of flavourings in each product varied, although most had menthol, carvone, linalool and limonene.
18C.4.3 Menthol, menthol derivatives and synthetic coolants
Mint or menthol flavours are very common in nicotine pouches and other oral nicotine products. They are produced by addition of the chemical menthol, or derivatives of menthol, or plant extracts such as peppermint that contain these chemicals. These additives are also common in other tobacco products, including smokeless tobacco (see Section 12.7). Menthol also has analgesic properties, reducing the feeling of pain in the upper airways that is caused by the harsh taste of nicotine in tobacco smoke.35 In this way, menthol can mask harshness of smoke, facilitating smoking in people who would otherwise be irritated by it.35 Menthol may be added to oral nicotine products to contribute to the flavour and mask the harshness of nicotine in these products.
Commercial names of oral nicotine products with menthol/mint flavour include the words wintergreen, ice cool, menthol and peppermint.33 A study of 48 different pouches bought in Germany found the presence of menthol and menthol derivatives that may produce these flavours and cooling effects.12 These menthol derivatives include isomenthol, isomenthyl acetate, neoisomenthol, and pulegone. These chemicals may have been additives (added on purpose), or impurities of mint extracts.12 Isomenthyl acetate is not an approved flavouring agent in the EU. Pulegone was withdrawn from recommended use as a food additive by the FDA in 2018.36 A study of five different pouches of different flavour description showed that menthol was present in each of these, despite only one indicating menthol as a flavour descriptor: the brands described as spice-chilli, tobacco, cola and energy all contained menthol.34
WS-3 is a synthetic chemical that imparts a cooling effect and has also been detected in some nicotine pouches.37 WS-3 and similar synthetic chemicals have an analgesic cooling effect via the same mechanisms that menthol uses, but without a notable flavouring effect. WS-3 is used as an additive in e-liquids and some tobacco products. As it is non-flavoured, it is likely that tobacco companies are using synthetic chemicals such as WS-3 to produce a cooling effect in products sold in jurisdictions that have a menthol ban where legislation does not extend to menthol analogues. See Sections 18.5.3.1 and 12.7.7.4 for more information about synthetic cooling agents.
18C.4.4 Other chemicals in oral nicotine products
According to their labels, oral nicotine pouches contain numerous different chemicals. In the list below, these have been classified into their likely functional roles by a study of products sold in Germany:12
- Sweeteners such as xylitol, sucralose and stevia
- Humectants such as propylene glycol, glycerol and agar agar
- Fillers such as cellulose and fibres from eucalyptus and pine trees
- Acidity regulators such as sodium carbonate and citric acid
- Salt such as rock salt
- Thickeners such as xanthan gum and guar gum
- Preservatives such as potassium sorbate
- Herbs such as black tea, ashwagandha and guarana
- Flavour enhancers such as β-Cyclodextrine
- Stabilisers such as hydroxypropyl cellulose and gum arabic
- Vegetable oil and water
A comprehensive study of the chemicals present in 48 different pouch types bought in Germany found a total of 186 chemicals, with a mean of 17 chemical types per pouch. This study found numerous chemicals, aside from those listed above. Most of these were likely to be additives rather than contaminants. These include caffeine, eugenol (a chemical from cloves that is the main flavouring in kreteks; see Section 3.27.6), benzoic acid (which may be used to form nicotine salts, see Section 18.5.3.1), saccharin (an undisclosed artificial sweetener), salicylic acid (a plant hormone with some skin healing properties) and some chemicals with toxicity concerns, discussed below.12
The artificial sweeteners acesulfame potassium and sucralose have been found in oral nicotine products, including those marketed as "unflavoured" or "flavour ban approved".38 Products with higher nicotine levels have higher levels of these sweeteners, indicating that they might play a role in masking the harsh taste of nicotine.38
18C.4.5 Contaminants and toxic chemicals
Contaminants in oral nicotine products may arise from contamination of the chemicals and flavouring mixes used to make the products, or from chemical reactions between these additives during storage. Given that many of these products contain nicotine isolated from tobacco plants, other constituents of tobacco have the potential to contaminate products made with nicotine. Nicotine replacement products are made from pharmaceutical-grade nicotine, which is highly purified to remove toxic contaminants. However, there is no such regulation of the nicotine and contaminants found in oral nicotine products. The higher expense of pharmaceutical-grade nicotine is likely to discourage its use by the manufacturers of nicotine pouches and other non-pharmaceutical products.14,15
Tobacco-specific nitrosamines (TSNAs) are carcinogenic (cancer-causing) chemicals derived from nicotine and found only in tobacco products (see Section 12.4.3.7). They are suspected causes of mouth, lung and oesophagus cancer and other types of cancer in people who smoke.6 Out of 44 tested nicotine pouches sold in Germany, 26 were found to have detectable amounts of TSNAs.2 The TSNAs called N-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) were detected in multiple nicotine pouch types.2
In a comprehensive study of the chemicals present in 48 nicotine pouches sold in Germany, three chemicals were detected that were designated as possible causes of cancer (Group 2B) by the International Agency for Research on Cancer (IARC). These chemicals are:12
- Methyl eugenol, found in 4 products, which induces liver tumours in rodents,
- Benzophenone, found in 1 product, which induces liver and kidney tumours in rodents,
- b-myrcene, which may induce liver and kidney tumours in rodents.
All three of these chemicals were withdrawn from recommended use as food additives by the FDA in 2018.36
18C.4.6 Biomarkers of exposure to chemicals from oral nicotine products
Unfortunately, no independent researchers to date have published studies of biomarkers of exposure to chemicals from oral nicotine products; all studies published so far have been conducted by tobacco companies, during testing of their own products.
One study of people who switched from tobacco smoking to sole use of oral nicotine products (discs/chews) showed a reduction in urinary biomarkers of exposure. These people had lower levels of tobacco-specific nitrosamines (TSNAs) as measured seven days after switching.39 However, urinary biomarkers of exposure to TSNAs remained detectable in the urine of people who had made this switch. These chemicals are only found in tobacco and their presence in the body indicates exposure to tobacco. In the case of sole use of oral nicotine products, urinary TSNAs may come from those contaminating the nicotine used to make these products. However, exposure to secondhand smoke or residual TSNAs from former smoking behaviour may also contribute to the TSNA biomarkers in the urine of oral nicotine users.
Related reading
Relevant news and research
Find out about recent news items and research on this topic (Last updated December 2024)
Read more on this topic
Test your knowledge
References
1. Stanfill S, Tran H, Tyx R, Fernandez C, Zhu W, et al. Characterization of total and unprotonated (free) nicotine content of nicotine pouch products. Nicotine & Tobacco Research, 2021; 23(9):1590-6. Available from: https://www.ncbi.nlm.nih.gov/pubmed/34233354
2. Mallock N, Schulz T, Malke S, Dreiack N, Laux P, et al. Levels of nicotine and tobacco-specific nitrosamines in oral nicotine pouches. Tobacco Control, 2024; 33(2):193-9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/38378209
3. Koszowski B, Viray LC, Stanfill SB, Lisko JG, Rosenberry ZR, et al. Nicotine delivery and pharmacologic response from Verve, an oral nicotine delivery product. Pharmacology Biochemistry and Behavior, 2015; 136:1-6. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26096037
4. German Federal Institute for Risk Assessment (BfR). Health risk assessment of nicotine pouches. 2022. Available from: https://mobil.bfr.bund.de/cm/349/health-risk-assessment-of-nicotine-pouches.pdf.
5. Tran H, Tyx RE, Valentin L, Mahoney M, Stanfill S, et al. Total and unprotonated (freebase) nicotine content in new types of oral 'tobacco-free' nicotine products. Tobacco Control, 2024. Available from: https://www.ncbi.nlm.nih.gov/pubmed/39542719
6. US Department of Health and Human Services, A report of the Surgeon General: How tobacco smoke causes disease.: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53017/.
7. Ebajemito JK, McEwan M, Gale N, Camacho OM, Hardie G, et al. A randomised controlled single-centre open-label pharmacokinetic study to examine various approaches of nicotine delivery using electronic cigarettes. Scientific Reports, 2020; 10(1):19980. Available from: https://www.ncbi.nlm.nih.gov/pubmed/33235307
8. van Amsterdam J, Sleijffers A, van Spiegel P, Blom R, Witte M, et al. Effect of ammonia in cigarette tobacco on nicotine absorption in human smokers. Food and Chemical Toxicology, 2011; 49(12):3025-30. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22001171
9. Gholap VV, Kosmider L, Golshahi L, and Halquist MS. Nicotine forms: why and how do they matter in nicotine delivery from electronic cigarettes? Expert Opinion on Drug Delivery, 2020; 17(12):1727-36. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32842785
10. Wilhelm J, Mishina E, Viray L, Paredes A, and Pickworth WB. The pH of smokeless tobacco determines nicotine buccal absorption: Results of a randomized crossover trial. Clinical Pharmacology and Therapeutics, 2022; 111(5):1066-74. Available from: https://www.ncbi.nlm.nih.gov/pubmed/34826137
11. Fant RV, Henningfield JE, Nelson RA, and Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tobacco Control, 1999; 8(4):387-92. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10629244
12. Mallock-Ohnesorg N, Rinaldi S, Malke S, Dreiack N, Pieper E, et al. Oral nicotine pouches with an aftertaste? Part 1: screening and initial toxicological assessment of flavorings and other ingredients. Archives of Toxicology, 2023; 97(9):2357-69. Available from: https://www.ncbi.nlm.nih.gov/pubmed/37389646
13. Chapman F, McDermott S, Rudd K, Taverner V, Stevenson M, et al. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic, pharmacodynamic and safety and tolerability profiles of tobacco-free oral nicotine pouches relative to cigarettes. Psychopharmacology (Berl), 2022; 239(9):2931-43. Available from: https://www.ncbi.nlm.nih.gov/pubmed/35732751
14. Jordt SE. Synthetic nicotine has arrived. Tobacco Control, 2023; 32(e1):e113-e7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/34493630
15. WHO study group on tobacco product regulation. Report on the scientific basis of tobacco product regulation: Ninth report of a WHO study group. WHO Technical Report Series, No. 1047., Licence: CC BY-NC-SA 3.0 IGO.Geneva: World Health Organization, 2023. Available from: https://www.who.int/publications/i/item/9789240079410.
16. Hellinghausen G, Lee JT, Weatherly CA, Lopez DA, and Armstrong DW. Evaluation of nicotine in tobacco-free-nicotine commercial products. Drug Testing and Analysis, 2017; 9(6):944-8. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27943582
17. Labstat. The difference between synthetic nicotine and natural nicotine. Available from: https://labstat.com/understanding-synthetic-nicotine-and-how-it-is-regulated-in-canada/.
18. Rossel S. Synthetic nicotine is gaining acceptance.: Tobacco reporter, 2019. Available from: https://tobaccoreporter.com/2019/12/01/mirror-image/.
19. Nisathar A, Chen H, Lei X, Zeng Z, and Chen J. Comparison of genotoxic impurities in extracted nicotine vs. synthetic nicotine. Frontiers in Chemistry, 2024; 12:1483868. Available from: https://www.ncbi.nlm.nih.gov/pubmed/39469415
20. Keller-Hamilton B, Curran H, Alalwan M, Hinton A, Brinkman MC, et al. Evaluating the role of nicotine stereoisomer on nicotine pouch abuse liability: A randomized crossover trial. Nicotine & Tobacco Research, 2024. Available from: https://www.ncbi.nlm.nih.gov/pubmed/38713545
21. Tobacco Tactics. Nicotine pouches. 2023. Available from: https://tobaccotactics.org/article/nicotine-pouches/
22. Erythropel HC, Jabba SV, Silinski P, Anastas PT, Krishnan-Sarin S, et al. Variability in constituents of e-cigarette products containing nicotine analogues. Journal of the American Medical Association, 2024; 332(9):753-5. Available from: https://www.ncbi.nlm.nih.gov/pubmed/39110443
23. Vagg R and Chapman S. Nicotine analogues: a review of tobacco industry research interests. Addiction, 2005; 100(5):701-12. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15847628
24. Jordt SE, Jabba SV, Zettler PJ, and Berman ML. Spree Bar, a vaping system delivering a synthetic nicotine analogue, marketed in the USA as 'PMTA exempt'. Tobacco Control, 2024. Available from: https://www.ncbi.nlm.nih.gov/pubmed/38499343
25. Jordt SE and Jabba SV. Introduction of nicotine analogue-containing oral pouch products in the United States. Tobacco Prevention & Cessation, 2024; 10. Available from: https://www.ncbi.nlm.nih.gov/pubmed/39588526
26. Wang DX, Booth H, Lerner-Marmarosh N, Osdene TS, and Abood LG. Structure–activity relationships for nicotine analogs comparing competition for [3H]nicotine binding and psychotropic potency. Drug Development Research, 1998; 45:10–16,:10–6. Available from: https://doi.org/10.1002/(SICI)1098-2299(199809)45:1<10::AID-DDR2>3.0.CO;2-GCitations
27. Dukat M, Fiedler W, Dumas D, Damaj I, Martin BR, et al. Pyrrolidine-modified and 6-substituted analogs of nicotine: A structure—affinity investigation. European Journal of Medicinal Chemistry, 1996; 31(11):875-88. Available from: https://www.sciencedirect.com/science/article/pii/S0223523497898509
28. Qi H, Chang X, Wang K, Xu Q, Liu M, et al. Comparative analyses of transcriptome sequencing and carcinogenic exposure toxicity of nicotine and 6-methyl nicotine in human bronchial epithelial cells. Toxicology In Vitro, 2023; 93:105661. Available from: https://www.ncbi.nlm.nih.gov/pubmed/37586650
29. Effah F, Sun Y, Friedman A, and Rahman I. Emerging nicotine analog 6-methyl nicotine increases reactive oxygen species in aerosols and cytotoxicity in human bronchial epithelial cells. Toxicology Letters, 2025; 405:9-15. Available from: https://www.ncbi.nlm.nih.gov/pubmed/39894318
30. Pubchem. Nicotine, 6-methyl-. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/25768196.
31. Berman ML, Zettler PJ, and Jordt SE. Synthetic nicotine: Science, global legal landscape, and regulatory considerations. World Health Organization Technical Report Series, 2023; 1047:35-60. Available from: https://www.ncbi.nlm.nih.gov/pubmed/37745838
32. Tobacco partners. Letter to FDA: Urgent public health imperative to regulate nicotine analog products. 2024. Available from: https://www.lung.org/getmedia/2d597ec0-581d-4a1d-a49f-06d58b3e7f19/Tobacco-Partners-Letter-to-FDA-on-nicotine-analog-products-5-29-24.pdf.
33. Shaikh SB, Tung WC, Pang C, Lucas J, Li D, et al. Flavor classification/categorization and differential toxicity of oral nicotine pouches (ONPs) in oral gingival epithelial cells and bronchial epithelial cells. Toxics, 2022; 10(11). Available from: https://www.ncbi.nlm.nih.gov/pubmed/36355951
34. Rinaldi S, Pieper E, Schulz T, Zimmermann R, Luch A, et al. Oral nicotine pouches with an aftertaste? Part 2: in vitro toxicity in human gingival fibroblasts. Archives of Toxicology, 2023; 97(9):2343-56. Available from: https://www.ncbi.nlm.nih.gov/pubmed/37482550
35. Wickham RJ. The biological impact of menthol on tobacco dependence. Nicotine & Tobacco Research, 2020; 22(10):1676-84. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31867627
36. Food and Drug Administration. Food additive regulations; synthetic flavoring agents and adjuvants. Federal Register, 2018. Available from: https://www.federalregister.gov/documents/2018/10/09/2018-21807/food-additive-regulations-synthetic-flavoring-agents-and-adjuvants.
37. Jabba SV, Erythropel HC, Woodrow JG, Anastas PT, O'Malley S, et al. Synthetic cooling agent in oral nicotine pouch products marketed as 'Flavour-Ban Approved'. Tobacco Control, 2023. Available from: https://www.ncbi.nlm.nih.gov/pubmed/37380351
38. Jabba SV, Silinski P, Yang AY, Ouyang W, and Jordt SE. Artificial sweeteners in US-marketed oral nicotine pouch products: Correlation with nicotine contents and effects on product preference. Nicotine & Tobacco Research, 2024. Available from: https://pubmed.ncbi.nlm.nih.gov/39656927/
39. Edmiston J, Liu J, Wang J, and Sarkar M. A randomized, controlled study to assess biomarkers of exposure in adult smokers switching to oral nicotine products. The Journal of Clinical Pharmacology, 2022; 62(11):1445-58. Available from: https://www.ncbi.nlm.nih.gov/pubmed/35730535